
Comprehensive Safety in IVF Treatments
At the partner institutions of Versys Clinics-VIIN, both clinical practice and embryology laboratories follow the most advanced international quality assurance standards—such as ISO 15189, ISO 20387 (Biobanking), and Good Laboratory Practice (GLP). These safety systems ensure the secure handling of reproductive materials (oocytes, sperm, embryos), minimize the risk of technological and human errors, and provide full transparency and traceability throughout every step of the IVF process.
Every biological sample—whether it’s an oocyte, sperm cell, or embryo—is registered with a unique identifier. The Laboratory Information Management System (LIMS) allows for real-time tracking of each sample’s status (e.g., fertilization, cell division, cryopreservation), ensuring full traceability and identifiability. The dual verification system and electronic witnessing systems play a critical role in preventing sample mix-ups—an ethically and legally crucial aspect in reproductive medicine.
Structured checklists and standardized workflow steps in the IVF laboratory ensure that every procedure is meticulously documented, fully traceable, and compliant with international standards. Laboratory operations are continuously monitored using key performance indicators (KPIs)—such as fertilization rate, embryo cleavage rate, cryosurvival rate, and the proportion of transferrable embryos—enabling clinics to evaluate and improve their performance consistently.
Among the latest technological advancements is the use of artificial intelligence (AI) and image-based embryo grading algorithms, which analyze the morphology and kinetics of embryos in real time, helping select the most viable embryos using predictive models. AI is also used for monitoring environmental conditions—such as temperature, CO₂/O₂ levels—inside incubators and cryostorage devices, allowing immediate intervention in case of any deviation.
Examples of Applied Quality Assurance Systems
1. Electronic Sample Identification – Witnessing System

What is it?
Electronic witnessing uses RFID (radio frequency identification) technology. Every oocyte, sperm, and embryo container is labeled with a unique RFID tag that communicates with a reader via radio waves. This ensures precise sample identification and tracking throughout all laboratory steps, minimizing the risk of human error and sample mix-ups.
Error Prevention
Prevents the possibility of sample confusion or switching
Complete Documentation
Every step is recorded, along with the identity of the staff and their actions
Couple-Specific Processing
Only samples from the same couple can be processed together
Automatic Alerts
The system alerts users to any inconsistencies, eliminating manual errors
2. Biometric Identification – Tomorrow Life Sciences Freezer System

What is it?
Developed by Tomorrow Life Sciences, this cryopreservation system uses biometric identification—such as iris recognition or other unique identifiers—during the vitrification (ultrarapid freezing) process.
3. Single Embryo Transfer (SET)
During IVF treatments, we not only use modern safety systems in the laboratory, but also strive for the safest possible solution during implantation (embryo transfer).
What is Single Embryo Transfer (SET)?
SET refers to the practice of transferring only one embryo into the uterus during an IVF cycle.
Why is it important?
SET significantly reduces the risk of multiple pregnancies (twins, triplets):
Transferring multiple embryos increases the chance of twin or triplet pregnancies, which can present serious health risks for both the mother and the babies. A single embryo transfer carries only about a 1% risk of twin pregnancy.
Risks associated with multiple pregnancies:
Multiple pregnancies require increased medical attention due to higher complication risks. The most common dangers include:
- Preterm birth
- Gestational diabetes
- High blood pressure
- Preeclampsia
- Need for C-section
- Twin-to-twin transfusion syndrome (TTTS)
- Developmental abnormalities
- Placenta previa
- Placental abruption
- Intrauterine growth restriction (IUGR)
- Iron-deficiency anemia
- Excess or deficient amniotic fluid
- Increased emotional stress
- More frequent hospital monitoring
Many believe that transferring two embryos increases the chance of a successful pregnancy. However, the truth is more nuanced—and your and your child’s health always comes first.
The following diagram illustrates why we strive for single embryo transfer when possible:
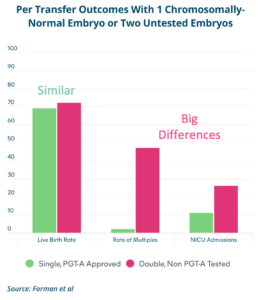
1. Live Birth Rate Remains the Same
When transferring a genetically tested (euploid) embryo, the success rate is similar to transferring two untested embryos.
2. Multiple Pregnancy Risk Drops Significantly
Transferring two embryos greatly increases the chance of a twin pregnancy, which introduces additional risks for both mother and babies.
3. Reduced Need for Neonatal Intensive Care
Twins or higher-order multiples are more likely to require care in a NICU due to premature birth or complications.
Important! Versys Clinics was the first in Hungary to introduce Single Embryo Transfer (SET) as a clinical protocol in 2016. This approach is not only scientifically proven, but also represents one of the safest choices to protect both your health and your future child’s well-being.
4. OHSS-Free IVF Clinic
An OHSS-free clinic follows a modern IVF treatment philosophy aimed at preventing Ovarian Hyperstimulation Syndrome (OHSS), a potential complication of ovarian stimulation during IVF.
This approach involves segmentation of the IVF process and the use of specialized stimulation and embryo-handling protocols.
What is OHSS (Ovarian Hyperstimulation Syndrome)?
OHSS is a complication caused by an exaggerated ovarian response, where ovaries become enlarged and fluid accumulates in the abdomen.
Symptoms:
Mild: bloating, abdominal discomfort, nausea.
Severe: intense abdominal pain, shortness of breath, blood clots, rapid weight gain.
Rarely: life-threatening condition.
OHSS-Free Protocol Components:
1. Segmented IVF Process
Treatments are divided into distinct stages, allowing for better control and safety.
2. GnRH Antagonist Protocol
GnRH agonist triggers final oocyte maturation instead of hCG, reducing OHSS risk.
3. Cryopreservation (Vitrification)
Oocytes or embryos are frozen instead of transferred immediately.
4. Frozen Embryo Transfer (FET)
Embryos are transferred in a later cycle when conditions are optimal.
Why is this approach beneficial?
5. Double Eye Witnessing – Dual Verification Protocols

What is it?
Double Eye Witnessing is a rigorous verification protocol applied at every critical point in IVF laboratory procedures. Two embryologists, working independently, must verify and approve every key step—such as oocyte retrieval, sample identification, processing, storage, and embryo selection.
Why is it important?
This protocol is essential for preventing human error, as it significantly reduces the risk of mistakes during sample identification and handling by requiring independent cross-checks by two professionals working on every critical laboratory procedure.
Your Safety is Our Priority
These comprehensive safety protocols ensure that every aspect of your IVF treatment meets the highest international standards, providing you with the safest and most effective path to parenthood.

